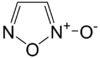
Furoxan
|
| Names
|
| Preferred IUPAC name
|
| Other names
Furazan N-oxide; Furazan 2-oxide
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
| MeSH
|
C528141
|
|
|
|
|
|
|
InChI=1S/C2H2N2O2/c5-4-2-1-3-6-4/h1-2H  N NKey: KCMCIQNPQUSQKQ-UHFFFAOYSA-N  N NInChI=1/C2H2N2O2/c5-4-2-1-3-6-4/h1-2H Key: KCMCIQNPQUSQKQ-UHFFFAOYAH
|
|
|
| Properties
|
|
|
C2H2N2O2
|
| Molar mass
|
86.050 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
Furoxan or 1,2,5-oxadiazole 2-oxide is a heterocycle of the isoxazole family and an amine oxide derivative of furazan. It is a nitric oxide donor.[1] As such, furoxan and its derivatives are actively researched as potential new drugs (Ipramidil) and insensitive high density explosives (4,4’-Dinitro-3,3’-diazenofuroxan).
Furoxanes can be formed by dimerization of nitrile oxides.
References
|
|---|
| Forms | |
|---|
| Targets | |
|---|
NO donors
(prodrugs) | |
|---|
Enzyme
(inhibitors) | |
|---|
| Others |
- Indirect/downstream NO modulators: ACE inhibitors/AT-II receptor antagonists (e.g., captopril, losartan)
- ETB receptor antagonists (e.g., bosentan)
- L-Type calcium channel blockers (e.g., dihydropyridines: nifedipine)
- Nebivolol (beta blocker)
- PDE5 inhibitors (e.g., sildenafil)
- non-selective PDE inhibitors (e.g., caffeine)
- PDE9 inhibitors (e.g., paraxanthine)
- cGMP preferring PDE inhibitors (e.g., sildenafil, paraxanthine, tadalafil)
- Statins (e.g., simvastatin)
|
|---|
|
