Chemical compound
Pharmaceutical compound
Iproheptine , also known as N -isopropyl-1,5-dimethylhexylamineN -isopropyloctodrineMetron and Susat , is a nasal decongestant which has been marketed in Japan .[ 1] [ 2] [ 3] vasoconstrictor and antihistamine .[ 1] [ 2] [ 3] over-the-counter in Japan.[ 4]
Pharmacology
Pharmacodynamics
Iproheptine is described as a decongestant , vasoconstrictor , and antihistamine .[ 1] [ 2] [ 3] pharmacology was characterized in a series of several preclinical studies published in the 1960s.[ 5] [ 6] [ 7] [ 8] [ 9] [ 10]
The drug was found to have anticholinergic - and antihistamine-like effects that were described as more potent than those of ephedrine .[ 6] [ 8] [ 10] hypotensive and cardiac inhibitive actions that made it differ from other known alkylamine and arylalkylamine sympathomimetics .[ 6] [ 8] blood vessels , pupils , and saliva secretion were all said to be very weak.[ 6] bronchodilation , vasoconstriction , and hemostasis similarly to ephedrine or methoxyphenamine .[ 7] [ 10] hexobarbital -induced sleep .[ 7] antidepressant - or stimulant -like effect in the forced swim test (FST).[ 9]
Close analogues of iproheptine, such as methylhexanamine and tuaminoheptane , are known to act as norepinephrine and/or dopamine releasing agents by interacting with the monoamine transporters , and this is thought to underlie their sympathomimetic and stimulant effects.[ 11] [ 12] [ 13] [ 14] [ 15] [ 16]
Pharmacokinetics
In contrast to arylalkylamines like phenethylamines and tryptamines , iproheptine is not metabolized by monoamine oxidase (MAO).[ 9]
Chemistry
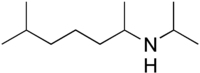
Iproheptine, also known as N -isopropyl-1,5-dimethylhexylamine or as N -isopropyloctodrine, is an alkylamine and the N -isopropyl derivative of octodrine (2-amino-6-methylheptane or 1,5-dimethylhexylamine (1,5-DMHA)).[ 1] [ 2] [ 3]
Aside from octodrine, it is also closely structurally related to other alkylamines, including 1,3-dimethylbutylamine (1,3-DMBA), 1,4-dimethylamylamine (1,4-DMAA), heptaminol (2-methyl-6-amino-2-heptanol), isometheptene (2-methyl-6-methylamino-2-heptene), methylhexanamine (1,3-dimethylamylamine (1,3-DMAA)), and tuaminoheptane (tuamine; 2-aminoheptane or 1-methylhexylamine).[ 1] [ 2] [ 3]
Iproheptine shows structural similarity to what would be 3- or 4-methyl-N -isopropylamphetamine, but with the equivalent of the phenyl ring open and incomplete (i.e., missing two carbon atoms , saturated , and the carbons not connected to form a ring).[ 17] [ 1]
The predicted log P (XLogP3 ) of iproheptine is 3.6.[ 17]
History
Iproheptine was first described in the scientific literature by 1960[ 5] [ 6] patented by 1962.[ 1] Japan in 2004.[ 2]
Society and culture
Names
Iproheptine is the generic name of the drug and its INN Tooltip International Nonproprietary Name .[ 1] [ 2] hydrochloride salt , its generic name is iproheptine hydrochloride and this is its JAN Tooltip Japanese Accepted Name .[ 2] Metron and Susat (both as the hydrochloride salt).[ 1] [ 2]
Availability
Iproheptine appears to have been marketed only in Japan .[ 2] over-the-counter in this country.[ 4]
See also
References
^ a b c d e f g h i Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies ISBN 978-1-4757-2085-3 . Retrieved 30 August 2024 . ^ a b c d e f g h i j Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory ISBN 978-3-88763-101-7 . Retrieved 30 August 2024 . ^ a b c d e Milne GW (2018). Drugs: Synonyms and Properties ISBN 978-1-351-78990-5 . Retrieved 30 August 2024 . Iproheptine 13946-02-6 6243 CHAN N-Isopropyl-1,5-dimethylhexylamine. Metron; Metron S. Antihistaminic. ^ a b "KEGG DRUG: Iproheptine Hydrochloride" . GenomeNet . Retrieved 30 August 2024 .^ a b Ota Y, Otani G, Enomoto R (1960). "Pharmacological Studies on Alkylaminoheptane Derivatives. I: Tracheal Muscle Spasmolytic Action of N-Alkyl-1, 5-dimethylhexylamine Derivatives" . Yakugaku Zasshi . 80 (9): 1153–1155. doi :10.1248/yakushi1947.80.9_1153 ISSN 0031-6903 . ^ a b c d e Ota Y, Enomoto R, Ishiguro Y (1960). "Pharmacological Studies on Alkylaminoheptane Derivatives. II: Pharmacological Action of N-Isopropyl-1, 5-dimethylhexylamine Hydrochloride. (1)" . Yakugaku Zasshi . 80 (9): 1156–1159. doi :10.1248/yakushi1947.80.9_1156 ISSN 0031-6903 . ^ a b c Ota Y, Fuchibe K, Takahashi M (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. III: Pharmacological Action of N-Isopropyl-1, 5-dimethylhexylamine Hydrochloride. (2)" . Yakugaku Zasshi . 81 (3): 394–402. doi :10.1248/yakushi1947.81.3_394 ISSN 0031-6903 . ^ a b c Ota Y (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. IV: Blood Pressor, Antispasmodic and Capillary Permeability Inhibiting Action of N-Alkyl-1, 5-dimethylhexylamine Derivatives" . Yakugaku Zasshi . 81 (3): 403–407. doi :10.1248/yakushi1947.81.3_403 ISSN 0031-6903 . ^ a b c Ota Y, Watabe M, Takahashi M (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. V: Pharmacological Action of N-isopropyl-1, 5-dimethylhexylamine Hydrochloride. (3)" . Yakugaku Zasshi . 81 (3): 407–414. doi :10.1248/yakushi1947.81.3_407 ISSN 0031-6903 . ^ a b c Ota Y (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. VI: Pharmacological Action of N-Isopropyl-1, 5-dimethyl-hexylamine Hydrochloride. (4)" . Yakugaku Zasshi . 81 (3): 415–420. doi :10.1248/yakushi1947.81.3_415 ISSN 0031-6903 . ^ Small C, Cheng MH, Belay SS, Bulloch SL, Zimmerman B, Sorkin A, et al. (August 2023). "The Alkylamine Stimulant 1,3-Dimethylamylamine Exhibits Substrate-Like Regulation of Dopamine Transporter Function and Localization" . J Pharmacol Exp Ther . 386 (2): 266–273. doi :10.1124/jpet.122.001573 . PMC 10353075 PMID 37348963 . ^ Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)" . Br J Pharmacol . 154 (3): 606–622. doi :10.1038/bjp.2008.124 . PMC 2439527 PMID 18500382 . ^ Delicado EG, Fideu MD, Miras-Portugal MT, Pourrias B, Aunis D (August 1990). "Effect of tuamine, heptaminol and two analogues on uptake and release of catecholamines in cultured chromaffin cells". Biochem Pharmacol . 40 (4): 821–825. doi :10.1016/0006-2952(90)90322-c . PMID 2386550 . ^ Alsufyani HA, Docherty JR (January 2019). "Methylhexaneamine causes tachycardia and pressor responses indirectly by releasing noradrenaline in the rat". Eur J Pharmacol . 843 : 121–125. doi :10.1016/j.ejphar.2018.10.047 . PMID 30395850 . ^ Schlessinger A, Geier E, Fan H, Irwin JJ, Shoichet BK, Giacomini KM, et al. (September 2011). "Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET" . Proc Natl Acad Sci U S A . 108 (38): 15810–15815. doi :10.1073/pnas.1106030108 . PMC 3179104 PMID 21885739 . ^ Rickli A, Hoener MC, Liechti ME (September 2019). "Pharmacological profiles of compounds in preworkout supplements ("boosters")". Eur J Pharmacol . 859 : 172515. doi :10.1016/j.ejphar.2019.172515 . PMID 31265842 . ^ a b "Iproheptine" . PubChem . Retrieved 8 December 2024 .