Norepinephrine (medication) Therapeutic use of norepinephrine
This article is about the medication. For this substance as a naturally occurring hormone, see
Norepinephrine .
Pharmaceutical compound
Norepinephrine Trade names Levarterenol, Levophed, Norepin, other Other names NoradrenalineR )-(–)-Norepinephrinel -1-(3,4-Dihydroxyphenyl)-2-aminoethanol AHFS /Drugs.com Monograph License data
Pregnancy Routes of Intravenous Drug class Adrenergic receptor agonist ; Sympathomimetic ATC code Source tissues Locus coeruleus ; sympathetic nervous system ; adrenal medulla Target tissues System-wide Receptors α1 , α2 , β1 , β3 Agonists Sympathomimetic drugs , clonidine , isoprenaline Antagonists Tricyclic antidepressants , Beta blockers , antipsychotics Metabolism MAO-A ; COMT Legal status
AU :S4 (Prescription only)CA OTC UK :POM (Prescription only)US :℞-only
Metabolism MAO-A ; COMT Excretion Urine (84–96%)
4-[(1R )-2-amino-1-hydroxyethyl]benzene-1,2-diol
CAS Number PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEBI ChEMBL Formula C 8 H 11 N O 3 Molar mass −1 3D model (JSmol ) Density 1.397±0.06 g/cm3 Melting point 217 °C (423 °F) (decomposes ) Boiling point 442.6 °C (828.7 °F) ±40.0°C
InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1
Y Key:SFLSHLFXELFNJZ-QMMMGPOBSA-N
Y (verify)
Norepinephrine , also known as noradrenaline and sold under the brand name Levophed among others, is a medication used to treat people with very low blood pressure .[ 2] sepsis if low blood pressure does not improve following intravenous fluids .[ 3] hormone and neurotransmitter norepinephrine .[ 2] slow injection into a vein .[ 2]
Common side effects include headache, slow heart rate, and anxiety .[ 2] irregular heartbeat .[ 2] limb ischemia .[ 2] phentolamine in the area affected may improve outcomes.[ 2] alpha adrenergic receptors .[ 2]
Norepinephrine was discovered in 1946 and was approved for medical use in the United States in 1950.[ 2] [ 4] generic medication .[ 2]
Medical uses
Norepinephrine is used mainly as a sympathomimetic drug to treat people in vasodilatory shock states such as septic shock and neurogenic shock , while showing fewer adverse side-effects compared to dopamine treatment.[ 5] [ 6]
Pharmacology
Mechanism of action
It stimulates α1 and α2 adrenergic receptors to cause blood vessel contraction , thus increases peripheral vascular resistance and resulted in increased blood pressure . This effect also reduces the blood supply to gastrointestinal tract and kidneys. Norepinephrine acts on beta-1 adrenergic receptors , causing increase in heart rate and cardiac output .[ 7] baroreceptor response to the rise in blood pressure as well as enhanced vagal tone ultimately result in a sustained decrease in heart rate.[ 8] [ 9]
Pharmacokinetics
Norepinephrine does not cross the blood–brain barrier under normal circumstances and hence is a peripherally selective drug .[ 10]
Chemistry
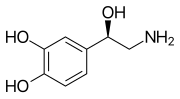
Norepinephrine, or noradrenaline, also known as 3,4,β-trihydroxyphenethylamine, is a substituted phenethylamine and catecholamine . It is the N -demethylated analogue of epinephrine (adrenaline; 3,4,β-trihydroxy-N -methylphenethylamine) and the β-hydroxylated analogue of dopamine (3,4-dihydroxyphenethylamine).
Society and culture
Names
Norepinephrine is the generic name of the drug and its INN Tooltip International Nonproprietary Name , while noradrenaline is its BAN Tooltip British Approved Name .[ 11] [ 12]
References
^ Andersen AM (1975). "Structural studies of metabolic products of dopamine. IV. Crystal and molecular structure of (-)-noradrenaline" . Acta Chemica Scandinavica B . 29 (8): 871–876. doi :10.3891/acta.chem.scand.29b-0871 PMID 1202890 . ^ a b c d e f g h i j "Norepinephrine Bitartrate" . The American Society of Health-System Pharmacists. Archived from the original on 26 March 2017. Retrieved 26 March 2017 .^ Latifi R (2016). Surgical Decision Making: Beyond the Evidence Based Surgery ISBN 9783319298245 Archived from the original on 2017-03-27. ^ Encyclopedia of the Neurological Sciences ISBN 9780123851581 Archived from the original on 2017-03-27.^ Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. (March 2017). "Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016" . Critical Care Medicine . 45 (3): 486–552. doi :10.1097/CCM.0000000000002255 . hdl :10281/267577 PMID 28098591 . S2CID 52827184 . We recommend norepinephrine as the first-choice vasopressor (strong recommendation, moderate quality of evidence). ^ De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. (March 2010). "Comparison of dopamine and norepinephrine in the treatment of shock" . The New England Journal of Medicine . 362 (9): 779–789. doi :10.1056/nejmoa0907118 PMID 20200382 . ^ Moore JI (6 December 2012). Pharmacology ISBN 9781468405248 . Retrieved 19 November 2017 . ^ Klabunde RE (7 December 2022). "Circulating Catecholamines" . CV Physiology . Retrieved 2019-02-27 . ^ Pollard S, Edwin SB, Alaniz C (July 2015). "Vasopressor and Inotropic Management Of Patients With Septic Shock" . P & T . 40 (7): 438–450. PMC 4495871 PMID 26185405 . ^ Froese L, Dian J, Gomez A, Unger B, Zeiler FA (October 2020). "The cerebrovascular response to norepinephrine: A scoping systematic review of the animal and human literature" . Pharmacol Res Perspect . 8 (5): e00655. doi :10.1002/prp2.655 . PMC 7510331 PMID 32965778 . ^ Elks, J. (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies ISBN 978-1-4757-2085-3 . Retrieved 31 August 2024 . ^ Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory ISBN 978-3-88763-101-7 . Retrieved 31 August 2024 .
External links
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β