|
Trichomonas vaginalis
Trichomonas vaginalis is an anaerobic, flagellated protozoan parasite and the causative agent of a sexually transmitted disease called trichomoniasis. It is the most common pathogenic protozoan that infects humans in industrialized countries.[2] Infection rates in men and women are similar but women are usually symptomatic, while infections in men are usually asymptomatic. Transmission usually occurs via direct, skin-to-skin contact with an infected individual, most often through vaginal intercourse. It is estimated that 160 million cases of infection are acquired annually worldwide.[3] The estimates for North America alone are between 5 and 8 million new infections each year, with an estimated rate of asymptomatic cases as high as 50%.[4] Usually treatment consists of metronidazole and tinidazole.[5] Clinical   HistoryAlfred Francois Donné (1801–1878) was the first to describe a procedure to diagnose trichomoniasis through "the microscopic observation of motile protozoa in vaginal or cervical secretions" in 1836. He published this in the article entitled, "Animalcules observés dans les matières purulentes et le produit des sécrétions des organes génitaux de l'homme et de la femme" in the journal, Comptes rendus de l'Académie des sciences.[6] With it, he created the binomial name of the parasite as Trichomonas vaginalis.[7] 80 years after the initial discovery of the parasitic protozoan, Hohne declared Trichomoniasis as a clinical entity in 1916.[8] Signs and symptoms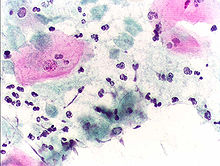 Most women (85%) and men (77%) with infected with T. vaginalis do not have symptoms. Half of these women can develop symptoms within 6 months and can have vaginal erythema, dyspareunia, dysuria, and vaginal discharge, which is often diffuse, malodorous, and yellow-green, along with itching in the genital region. “Strawberry cervix,” occurs in about 5% of women. In men, it can cause urethritis, epididymitis and prostatitis.[9] ComplicationsSome of the complications of Trichomonas vaginalis in women include: preterm delivery, low birth weight, and increased mortality as well as predisposing to human immunodeficiency virus infection, AIDS, and cervical cancer.[10] Trichomonas vaginalis can be seen in diverse locations within the body, such as," in the urinary tract, fallopian tubes, and pelvis and can cause pneumonia, bronchitis, and oral lesions."[11] Diagnosis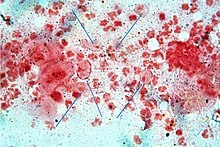 Classically, with a cervical smear, infected women may have a transparent "halo" around their superficial cell nucleus but more typically the organism itself is seen with a, "slight cyanophilic tinge, faint eccentric nuclei, and fine acidophilic granules."[12] It is unreliably detected by studying a genital discharge or with a cervical smear because of their low sensitivity. Trichomonas vaginalis is also routinely diagnosed via a wet mount, in which motility is observed. Currently, the most common method of diagnosis is via overnight culture,[13][14] with a sensitivity range of 75–95%.[15] Newer methods, such as rapid antigen testing and transcription-mediated amplification, have even greater sensitivity, but are not in widespread use.[15] TreatmentInfection is treated and cured with metronidazole[16] or tinidazole. The CDC recommends a one time dose of 2 grams of either metronidazole or tinidazole as the first-line treatment; the alternative treatment recommended is 500 milligrams of metronidazole, twice daily, for seven days if there is failure of the single-dose regimen.[17] Medication should be prescribed to any sexual partner(s) as well because they may be asymptomatic carriers.[18] Morphology Trichomonas vaginalis exists in only one morphological stage, a trophozoite, and cannot encyst (or form cysts.) This protozoan does not typically adhere to one shape, as in different conditions, the parasite has the ability to change. When in culture separate from the host, it usually displays a more "pear" or oval shaped morphology, but when present in a living host, specifically on the epithelial cells of the vaginal wall, the shape is more "amoeboid".[19] It is slightly larger than a white blood cell, measuring 9 × 7 μm. In both forms, Trichomonas vaginalis has five flagella – four protruding from the front or anterior of the parasite and the fifth on the back or posterior end. The functionality of the fifth flagellum is not known.[20] In addition, a barb-like axostyle projects opposite the four-flagella bundle. All of these flagella are connected to an "undulating" membrane.[20] The axostyle may be used for attachment to surfaces and may also cause the tissue damage seen in trichomoniasis.[21] The nucleus is usually elongated, and is located near the anterior end of the protozoan within the cytoplasm which contains many hydrogenosomes (closed-membrane organelle with the ability to produce both adenosine triphosphate and hydrogen while in anaerobic conditions.)[22] While Trichomonas vaginalis does not have a cyst form, the organism can survive for up to 24 hours in urine, semen, or even water samples. A nonmotile, round, pseudocystic form with internalized flagella has been observed under unfavorable conditions.[23] This form is generally regarded as a degenerate stage as opposed to a resistant form,[23] although viability of pseudocystic cells has been occasionally reported.[24] The ability to revert to trophozoite form, to reproduce and sustain infection has been described,[25] along with a microscopic cell staining technique to visually discern this elusive form.[26] MetabolismTrichomonas vaginalis is an anaerobe.[27] There is an absence of cytochrome C and mitochondria, thus making oxygen uptake and synthesis of adenosine triphosphate via oxidative phosphorylation difficult.[27] Although it contains no mitochondria, an analogous structure called a hydrogenosome, which is the site of fermentative oxidation of pyruvate, carries out many of the same metabolic processes. Carbohydrates, specifically those with alpha1,4- glycosidic linkages, are metabolized and eventually fermented to produce products such as acetate, lactate, malate, glycerol and CO2 under aerobic conditions. Hydrogen is produced under anaerobic conditions.[28] Outside the hydrogenosome, carbohydrate metabolism also occurs freely in the cytoplasm. The Embden-Meyerhof-Parnas pathway.[28] is used to convert glucose into phosphoenolpyruvate which ultimately becomes pyruvate. Virulence factorsAlthough Trichomonas vaginalis exists as a trophozoite in its infective form, its amoeboid form is also an important characteristic that adds to how well it is able to infect its host. The amoeboid form, which is pancake shaped, allows for greater surface area contact with epithelial cells of the vagina, cervix, urethra, and prostate. [29] The pseudocyst form is also a way in which the microbe can infect more efficiently, but this only induced when exposed to cold and other stressors.[29] These various forms are accompanied with differing protein phosphorylation profiles which are triggered by environmental pressures.[29] One of the hallmark features of Trichomonas vaginalis is the adherence factors that allow cervicovaginal epithelium colonization in women. The adherence that this organism illustrates is specific to vaginal epithelial cells being pH, time, and temperature dependent.[30] A variety of virulence factors mediate this process some of which are the microtubules, microfilaments, bacterial adhesins (4), and cysteine proteinases. The adhesins are four trichomonad enzymes called AP65, AP51, AP33, and AP23 that mediate the interaction of the parasite to the receptor molecules on vaginal epithelial cells.[31] The best characterized surface molecule associated with one of the four adhesins is called Trichomonas vaginalis lipoglycans.[29] This molecule is the most abundant on the surface of Trichomonas vaginalis, aids in sticking to vaginal epithelial cells, and can also influence how the human immune system responds, affecting inflammatory responses and macrophages in the body.[29] Cysteine proteinases may be another virulence factor because not only do these 30 kDa proteins bind to host cell surfaces but also may degrade extracellular matrix proteins like hemoglobin, fibronectin or collagen IV.[30] Genome sequencing and statisticsThe Trichomonas vaginalis genome is approximately 160 megabases in size[32] – ten times larger than predicted from earlier gel-based chromosome sizing.[33] (The human genome is ~3.5 gigabases by comparison.[34]) As much as two-thirds of the Trichomonas vaginalis sequence consists of repetitive and transposable elements, indicative of a recent drastic, evolutionarily expansion of the genome. The total number of predicted protein-coding genes is ~60,000, with the genome being around 65% repetitive (virus-like, transposon-like, retrotransposon-like, and unclassified repeats, all with high copy number and low polymorphism).[35] Approximately 26,000 of the protein-coding genes have been classed as 'evidence-supported' (similar either to known proteins, or to expressed sequence tags), while the remainder have no known function. [35]These extraordinary genome statistics are likely to change downward as the genome sequence, currently very fragmented due to the difficulty of ordering repetitive DNA, is assembled into chromosomes, and as more transcription data (expressed sequence tags, microarrays) accumulate. [35] TrichDB.org was launched as a free, public genomic data repository and retrieval service devoted to genome-scale trichomonad data. The site currently contains all of the Trichomonas vaginalis sequence project data, several expressed sequence tag libraries, and tools for data mining and display. [36] TrichDB is part of the EupathDB functional genomics database project funded by the National Institutes of Health and National Institute of Allergy and Infectious Diseases.[36] Genetic diversityHigh levels of genetic diversity were detected in Trichomonas vaginalis after phenotypic differences were discovered during clinical presentations. [37] Studies into the genetic diversity of Trichomonas vaginalis has shown that there are two distinct lineages of the parasite found worldwide; both lineages are represented evenly in field isolates. The two lineages differ in whether or not Trichomonas vaginalis virus infection is present. Trichomonas vaginalis virus infection is clinically relevant in that, it has an effect on parasite resistance to metronidazole, a first line drug treatment for human trichomoniasis.[38] Increased susceptibility to human immunodeficiency virusIn addition to inflammation that Trichomonas vaginalis causes, the parasite also causes lysis of epithelial cells and red blood cells in the area leading to more inflammation and disruption of the protective barrier usually provided by the epithelium. Having Trichomonas vaginalis also may increase the chances of the infected woman transmitting human immunodeficiency virus to her sexual partner(s).[39][40] EvolutionThe biology of Trichomonas vaginalis has implications for understanding the origin of sexual reproduction in eukaryotes. Trichomonas vaginalis is not known to undergo meiosis, a key stage of the eukaryotic sexual cycle. However, when Malik et al.[41] examined Trichomonas vaginalis for the presence of 29 genes known to function in meiosis, they found 27 homologous genes to the ones found in animals, fungi, plants and other protists, including eight of nine genes that are specific to meiosis in model organisms.[41] These findings suggest that Trichomonas vaginalis has the capability for meiotic recombination, and hence "parasexual" reproduction.[41] 21 of the 27 meiosis genes were also found in another parasite Giardia lamblia (also called Giardia intestinalis), indicating that these meiotic genes were present in a common ancestor of Trichomonas vaginalis and G. intestinalis.[41] Since these two species are descendants of lineages that are highly divergent among eukaryotes, these meiotic genes were likely present in a common ancestor of all eukaryotes.[41] See alsoReferences
Further reading
External links
|
||||||||||||||||||||||||
