|
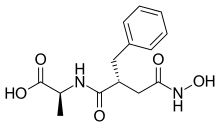
Kelatorphan
Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE).[1][2][3] In mice, with the intracerebroventricular co-administration of a 50 μg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier)[4] hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times.[1] Kelatorphan also displays potent antinociceptive effects alone,[5] and does not depress respiration, although at high doses it actually increases it.[4] See alsoReferences
|
||||||||||||||||||||||||||||||||||||||