|
Leu-enkephalin
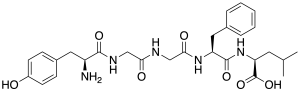
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans.[2][3] It is one of the two forms of enkephalin; the other is met-enkephalin.[2] The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine.[4] Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.[5][6] A nasal spray formulation of leu-enkephalin (developmental code names NES-100, NM-0127, NM-127, PES-200; proposed brand name Envelta) is under development by Virpax Pharmaceuticals for the treatment of pain and post-traumatic stress disorder (PTSD).[7] As of November 2023, it is up to the preclinical stage of development for these indications.[7] See alsoReferences
|
||||||||||||||||||||||||||||||||||||||||||